Hyperbaric Oxygen Treatment For long Coronavirus Disease-19: A Case Report
Background
Te coronavirus disease 2019 (COVID-19) pandemic has resulted in a growing population of individuals who experience a wide range of long lasting symptoms after recovery from the acute illness, referred to by several terms, including “post-COVID conditions” and “long COVID.” Te fve most common symptoms recognized post-COVID are fatigue (58%), headache (44%), cognitive impairment (27%), hair loss (25%), and dyspnea (24%) [1]. Two main biological sequelae of COVID-19 play roles in the pathogenesis of long COVID. Te frst is hyper- coagulability state characterized by increased risk of small- and large-vessel occlusion [2]. Te second is an uncontrolled continuous infammatory response [3]. Microinfarcts and neuroinfammation are important causes of brain hypoxia and can be responsible for the chronic unremitting neurocognitive decline in patients with long COVID [4]. One of the options to reverse hypoxia, reduce neuroinfammation, and induce neu- roplasticity is hyperbaric oxygen therapy (HBOT) [5].
In this article, we present the frst case report of previ- ously healthy, athletic individual who sufered from long- standing post-COVID syndrome treated successfully with HBOT.
Case presentation
A 55-year-old previously healthy Caucasian man suf fering from persistent unremitting symptoms of long COVID attended our clinic for evaluation. Te clinical presentation included memory problems, worsening of multitasking abilities, fatigue, low energy, breathless- ness, and reduced physical ftness, which all started after acute SARS-CoV-2 infection diagnosed 3 months before. He initially developed high-grade fever without chest pain, cough, or shortness of breath, on 21 January 2021. He was admitted to hospital because of dehydration on 30 January 2021 and was diagnosed with COVID-19 by reverse-transcription polymerase chain reaction (RT- PCR). During the hospital stay, he developed acute res- piratory syndrome due to pneumonitis and required supportive treatment with high-fow oxygen for 1 week. He was discharged from hospital on 16 February 2021. At discharge, he was stable with normal oxygen and no neurological defciencies were noted on physical exami- nation. In addition, 6 weeks after being diagnosed with COVID-19, he developed a pulmonary embolus and was treated with rivaroxaban. Prior to the SARS-CoV-2 infec- tion, he had been a healthy, high-functioning, and ath- letic individual.
Te baseline evaluation done at our clinic, 3 months after the acute infection, included brain magnetic reso- nance imaging (MRI) with perfusion and difusion tensor imaging (DTI), computerized neurocognitive evaluation, cardiopulmonary exercise test (CPET), and pulmonary function tests.
At baseline, the patient complained of shortness of breath with exercise as well as difculties with memory and multitasking that started after his COVID-19 illness.
Physical and neurological examination was normal. Brain MRI evaluation demonstrated reduced perfusion that correlated with the cognitive decline as detailed below. He was referred to hyperbaric oxygen therapy (HBOT) that included 60 sessions, 5 days per week. Each ses- sion included exposure to 90 minutes of 100% oxygen at 2 atmosphere absolute with 5-minute air breaks every 20 minutes.
Te patient started his frst HBOT on 19 April 2021 and fnished on 15 July 2021 without any signifcant side efects. After the frst fve sessions, he reported that his breathing had started to improve and that he no longer had muscle aches after exercise. After 15 sessions, he noted less fatigue and an improvement in his previous low energy. After 20 sessions, he noticed that his breath- ing and exercise capacity had returned to his capacity pre-SARS-CoV-2 infection, returning to running moun- tain trails. Additionally, he noted that his memory and multitasking ability returned to his pre-COVID-19 levels.
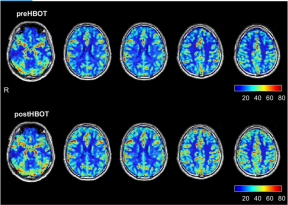
Te baseline brain MRI, prior to the HBOT, showed two small foci of signal alterations in the right and left parietal regions suggestive of early small vessel disease. In addition, there was a global decrease in the brain per- fusion. As detailed in Fig. 1 and Table 1, re-evaluation after HBOT (done 4 weeks after the last HBOT to avoid any potential intermediate efect) revealed a signifcant increase in brain perfusion. Tables 2 and 3 present the improvements in the brain microstructure as demon- strated by MRI–DTI.
Neurocognitive assessment was done using NeuroTrax full computerized testing battery to measure diferent aspects of brain function, such as memory, information processing speed, attention, and executive function, was done before and after HBOT. Te post-HBOT neurocog- nitive testing showed signifcant improvement in global memory with the most dominant efect being on nonver- bal memory, executive functions, attention, information procession speed, cognitive fexibility, and multitasking. Table 4 summarizes the pre- and post-HBOT scores in the diferent cognitive domains.
Physical capacity was evaluated by maximal cardiopul- monary exercise test (CPET) conducted on a COSMED treadmill using the Boston 5 protocol. Table 5 presents the pre- and post-HBOT physiological evaluated param- eters. As detailed, there was a 34% increase in the VO2 max from 3083 to 4130 mL per minute after HBOT. Te forced vital capacity (FVC) improved by 44% from 4.76 to 6.87 L, the forced expiratory volume (FEV) by 23% from 3.87 to 4.76 L, and peak fow measurement (PEF) by 20.2% from 10.17 to 12.22 L per second.
After receiving full information at the end of his post- HBOT evaluation, the patient signed an informed con- sent allowing publication of his medical information.
Fig. 1 Brain perfusion magnetic resonance imaging before and after hyperbaric oxygen therapy. The upper row represents brain perfusion 3 months after the acute infection, before hyperbaric oxygen therapy. The lower row represents the perfusion magnetic resonance imaging done after completing the hyperbaric oxygen therapy protocol.
Table 1 Brain blood fow changes before and after hyperbaric oxygen therapy
| Brain region Pre-HBOT Post-HBOT Change in % | |||
| White matter right (R) | 19.43 | 22.89 | 17.80 |
| White matter left (L) | 19.17 | 22.23 | 16 |
| Gray matter R | 32.34 | 38.6 | 19.40 |
| Gray matter L | 33.3 | 38.91 | 16.80 |
| Primary gustatory cortex R | 34.22 | 47.43 | 38.60 |
| Lateral postcentral gyrus R | 32.08 | 42.79 | 33.40 |
| Superior temporal gyrus R | 38.04 | 50.65 | 33.10 |
| Supramarginal gyrus R | 36.37 | 46.39 | 27.60 |
| Anterior cingulate cortex L | 40.16 | 50.61 | 26 |
| Inferior frontal gyrus L | 39.47 | 49.6 | 25.70 |
| Inferior frontal gyrus (Broca’s | 37.55 | 46.81 | 24.70 |
| area) R | |||
| Medial frontal gyrus R | 29.57 | 36.67 | 24 |
Discussion and conclusions
Here, we report the frst case of a patient with long COVID with cognitive and cardiorespiratory symptoms treated successfully by HBOT. Following treatment, he showed signifcant improvements in brain perfusion, white matter brain microstructure, and cognitive and cardiopulmonary function. Tis case report shows that HBOT has potential use for treatment of patients with long COVID who sufer from unremitting cognitive and physical functional decline.
Hypoxia plays an important role in the pathophysiol- ogy of long COVID. Systemic hypoxia could result from lung impairment, and organ-related hypoxia can develop because of vascular damage. Persisting lung function
Table 2 Magnetic resonance imaging–difusion tensor imaging fractional anisotropy changes before and after hyperbaric oxygen therapy
| Brain region Pre-HBOT Post-HBOT Change in % | ||||
| Superior fronto-occipital fasciculus L | 0.44 | 0.48 | 7.52 | |
| Cingulum (hippocampus) | R | 0.24 | 0.26 | 7.46 |
| Superior corona radiata L | 0.39 | 0.42 | 5.63 | |
| Body of corpus callosum | 0.43 | 0.45 | 5.39 | |
| Cingulum (hippocampus) | L | 0.23 | 0.24 | 4.59 |
| Corticospinal tract L | 0.37 | 0.38 | 3.49 | |
| External capsule L | 0.36 | 0.38 | 3.23 | |
| Superior corona radiata R | 0.43 | 0.44 | 3.21 | |
Fractional anisotropy (FA) is a measure used to evaluate white matter fber integrity, directionality, and order. A higher value of FA indicates better fber organization. DTI difusion tensor imaging
Table 3 Magnetic resonance imaging–difusion tensor imaging mean difusivity changes before and after hyperbaric oxygen therapy
| Brain region Pre-HBOT Post-HBOT Change in % | |||
| Medial lemniscus R | 1.3 | 1.24 | 4.72 |
| Superior longitudinal fascicu- lus L | 0.76 | 0.73 | 4.61 |
| Medial lemniscus L | 1.23 | 1.18 | 4.34 |
| Superior corona radiata L | 0.77 | 0.74 | 3.18 |
| Superior fronto-occipital
fasciculus L |
0.75 | 0.72 | 3.14 |
| Sagittal stratum L | 0.83 | 0.81 | 2.51 |
| Pontine crossing tract | 0.76 | 0.75 | 2.35 |
| Fornix L | 1.01 | 0.99 | 2.06 |
Mean difusivity (MD) is a measure used to evaluate white matter fber density. A lower value of MD indicates a higher density. DTI difusion tensor imaging.
Table 4 Cognitive scores before and after hyperbaric oxygen therapy
| Neurotrax | Pre-HBOT | Post-HBOT | Change in % |
| Global cognitive score | 93.3 | 99.4 | 6.5 |
| Memory | 98.8 | 105.8 | 7.1 |
| Nonverbal memory | 96.2 | 114 | 18.5 |
| Delayed nonverbal memory | 105.6 | 113.6 | 7.6 |
| Verbal memory | 92.1 | 94.5 | 2.6 |
| Delayed verbal memory | 101.3 | 101.3 | 0 |
| Executive function | 101.2 | 112.6 | 11.3 |
| Information processing speed | 74.6 | 80.8 | 8.3 |
| Attention | 87.9 | 92.1 | 4.8 |
| Motor skills | 104 | 105.6 | 1.5 |
Table 5 Physiological parameters before and after hyperbaric oxygen therapy
| Cardiopulmonary exercise test | |||
| VO2 max (mL/min) | 3083 | 4130 | 34 |
| VO2max/kg (mL/min/kg) | 31.5 | 42.4 | 34.6 |
| Lactic threshold (mL/min) | 2941 | 3439 | 16.9 |
| Respiratory threshold (mL/min) | 3103 | 4076 | 31.4 |
| Metabolic equivalent of task | 9 | 12.1 | 34.4 |
| (MET) | |||
| Maximal heart rate (bpm) | 155 | 164 | 5.8 |
| VO2/HR (mL per beat) | 19.9 | 25.2 | 26.6 |
| Pulmonary function tests | |||
| FVC (L) | 4.76 | 6.87 | 44.3 |
| FEV1 (L) | 3.87 | 4.76 | 23 |
| PEF (L/s) | 10.17 | 12.22 | 20.2 |
VO2max maximum rate of oxygen consumed during exercise, ml/min milliliter per minute, VO2max/kg maximum rate of oxygen consumed during exercise per kilogram, ml/min/Kg milliliters per minute per kilogram, MET metabolic equivalent of task, bpm heartbeats per minute, VO2/HR rate of oxygen consumed per heart rate, FVC forced vital capacity, L liters, FEV1 forced expiratory volume, PEF peak fow measurement, L/s liters per second.
impairment has been seen in patients who required sup- plemental oxygen during acute SARS-CoV-2 infection even 6 and 12 months after the acute infection [6]. Since brain functionality and regenerative capacity is sensitive to any decline in oxygen supply [7], long-term cognitive defcits correlate with the amount of oxygen needed to overcome the respiratory difculties [1]. With regard to organ-related ischemia, COVID-19 induced endothelial damage and hypercoagulation, which increases the risk of vascular dysfunction responsible for the high preva- lence of myocardial infarction, ischemic strokes, and pul- monary embolism [8]. In the presented case, the patient required supportive treatment with high-fow oxygen for 1 week during the acute illness, meaning he had sufered from systemic hypoxia with its consequent risk for long- term cognitive impairment due to anoxic brain damage. Moreover, 6 weeks after the acute infection, he developed a pulmonary embolus, representative of the endothe- lial dysfunction with additional exposure to systemic hypoxia. In addition, as demonstrated by the brain perfu- sion MRI, he had microvascular-related perfusion defects that correlated with his neurocognitive decline.
HBOT involves the inhalation of 100% oxygen at pres- sures exceeding 1 atmosphere absolute (ATA), thus enhancing the amount of oxygen dissolved in the body tissues. Even though many of the benefcial efects of HBOT can be explained by improvement of tissue oxy- genation, it is now understood that the combined action of hyperoxia and hyperbaric pressure triggers both oxy- gen- and pressure-sensitive genes, resulting in induction of regenerative processes including stem cell proliferation and mobilization with anti-apoptotic and anti-infam- matory factors, angiogenesis, and neurogenesis [9–12]. HBOT can induce neuroplasticity and improve cogni- tive function even years after the acute insult [13]. In the case presented of long COVID, HBOT improved cerebral blood fow to the malperfused brain regions (indicative of brain angiogenesis) and improved the integrity of brain microstructure (indicative of neurogenesis). Te correla- tion between the signifcant improvements demonstrated on brain imaging and the neurocognitive improvements indicates that most of the benefcial efects of HBOT are indeed related to its ability to induce neuroplasticity of the brain’s dysfunctional regions.
HBOT has been demonstrated to have benefcial efects on mitochondrial function, a crucial element of appropri- ate muscle function [12]. HBOT can also increase the number of proliferating and diferentiating satellite cells as well as the number of regenerated muscle fbers, and promote muscle strength [14]. Te newly intermittent repeated HBOT protocol was demonstrated to have the potential to improve lung function with respect to peak expiratory fow (PEF) and force vital capacity (FVC) [15]. In the presented patient, performance capacity of the car- diopulmonary system was evaluated using cardiopulmo- nary exercise test (CPET) and pulmonary function tests. HBOT induced a signifcant improvement of 34% in the maximal oxygen consumption capacity, an improvement of 34.4% in the maximal METs, and an increase of 16.9% in the lactic threshold. With regard to lung function, FVC was improved by 44.3%, and PEF by 20.2%. Tese meas- urable improvements correlated with the patient’s ability to regain his previous high athletic performance.
In this reported case, HBOT was initiated more than 3 months after the acute SARS-CoV-2 infection. Even though the symptoms persisted till the HBOT was ini- tiated and signifcant improvement began only after HBOT was initiated, it is possible that at least some of the clinical improvement could have occurred without HBOT. However, the abrupt signifcant improvement with full recovery after the chronic nature of the symp- toms, our understanding of the physiological efects of HBOT, and the objective measurements done on this patient support the relation between the treatment and the improvements seen. As this is only a case report, fur- ther prospective clinical trials are needed to gain a bet- ter understanding of the potential benefcial efects of HBOOT for patients with long COVID.
In summary, this article represents the first case report showing that long COVID can be treated with HBOT. Te benefcial efect of HBOT sheds additional light on the pathophysiology of this syndrome. As this is a sin- gle case report, further prospective randomized control studies are needed for the use of hyperbaric oxygen ther- apy in treating long COVID.
Abbreviations
HBOT: Hyperbaric oxygen therapy; MRI: Magnetic resonance imaging; DTI: Dif- fusion tensor imaging; VO2 max: Maximum rate of oxygen consumed during exercise; CPET: Cardiopulmonary exercise test; HR: Heart rate; Bpm: Heart beats per minute; FVC: Forced vital capacity; FEV1: Forced expiratory volume; PEF: Peak fow measurement.
Acknowledgements
Not applicable.
Authors’ contributions
AMB, ES, SE, and SK analyzed and interpreted the patient data regarding the MRI, perfusion, and DTI. AMB and SE analyzed and interpreted the patient data regarding the cardiopulmonary and pulmonary function tests. All authors read and approved the fnal manuscript.
Funding
No funding was received.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests
AMB, ZW, SK, MG, and UQ work for AVIV Clinics. ES works for AVIV Scientifc LTD. SE is a cofounder and shareholder at AVIV Scientifc LTD.
Received: 11 October 2021 Accepted: 21 January 2022, Published online: 15, February 2022
References
- Lopez-Leon S, et al. More than 50 long-term efects of COVID-19: a sys- tematic review and meta-analysis. Sci Rep. 2021;11(1):16144.
- LeviM, et al. Coagulation abnormalities and thrombosis in patients with COVID-19. Lancet Haematol. 2020;7(6):e438–40.
- MahmudpourM, et al. COVID-19 cytokine storm: the anger of infamma- tion. Cytokine. 2020;133: 155151.
- LiB, et al. Brain–immune interactions in perinatal hypoxic-ischemic brain injury. Prog Neurobiol. 2017;159:50–68.
- ShapiraR, et al. Hyperbaric oxygen therapy ameliorates pathophysiology of 3xTg-AD mouse model by attenuating neuroinfammation. Neurobiol Aging. 2018;62:105–19.
- HuangL, et al. 1-year outcomes in hospital survivors with COVID-19: a longitudinal cohort study. Lancet. 2021;398(10302):747–58.
- HadannyA, Efrati S. Oxygen—a limiting factor for brain recovery. Crit Care. 2015;19:307.
- KatsoularisI, et al. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: a self-controlled case series and matched cohort study. Lancet. 2021;398(10300):599–607.
- Pena-VillalobosI, et al. Hyperbaric oxygen increases stem cell prolifera- tion, angiogenesis and wound-healing ability of WJ-MSCs in diabetic mice. Front Physiol. 2018;9:995.
- CabigasBP, et al. Hyperoxic and hyperbaric-induced cardioprotection: role of nitric oxide synthase 3. Cardiovasc Res. 2006;72(1):143–51.
- GregorevicP, Lynch GS, Williams DA. Hyperbaric oxygen modulates antioxidant enzyme activity in rat skeletal muscles. Eur J Appl Physiol. 2001;86(1):24–7.
- ZhouZ, et al. Protection of mitochondrial function and improvement in cognitive recovery in rats treated with hyperbaric oxygen following lateral fuid-percussion injury. J Neurosurg. 2007;106(4):687–94.
- HadannyA, et al. Hyperbaric oxygen therapy improves neurocognitive functions of post-stroke patients—a retrospective analysis. Restor Neurol Neurosci. 2020;38(1):93–107.
- HorieM, et al. Enhancement of satellite cell diferentiation and functional recovery in injured skeletal muscle by hyperbaric oxygen treatment. J Appl Physiol. 2014;116(2):149–55.
- HadannyA, et al. Hyperbaric oxygen therapy effects on pulmonary func-tions: a prospective cohort study. BMC Pulm Med. 2019;19(1):148.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in pub- lished maps and institutional afliations.